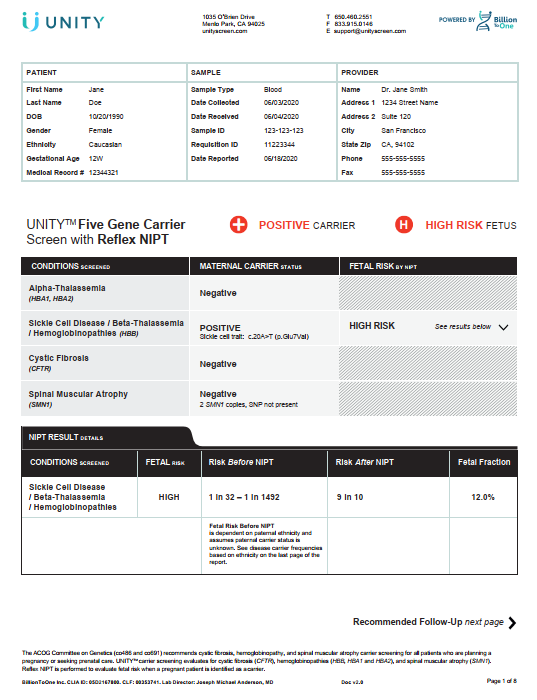
UNITY Test by BILLION TO ONE
UNITY screens your baby’s risk for these conditions without needing a paternal sample.
- Cystic fibrosis
- Spinal muscular atrophy
- Sickle cell disease
- Alpha & beta thalassemia
- Trisomy 21 (Down syndrome)
- Trisomy 18 (Edwards syndrome)
- Trisomy 13 (Patau syndrome)
- Sex chromosome aneuploidy

Aneuploidy screening and foetal sex is available for twin pregnancies. Fragile X carrier screening is also available as an add-on. Foetal sex is included unless opted out.
UNITY is one simple test
Small fragments of DNA related to your baby that made its way from the placenta into your bloodstream are analysed.
Using BillionToOne’s proprietary Quantitative Counting Template (QCT) technology, UNITY can assess your baby’s risk for recessively inherited conditions, changes in chromosome numbers and your baby’s RhD status.
What is the UNITY screening test?
Watch the following videos to learn more about the UNITY screening test.
What makes UNITY screen different from other NIPT?
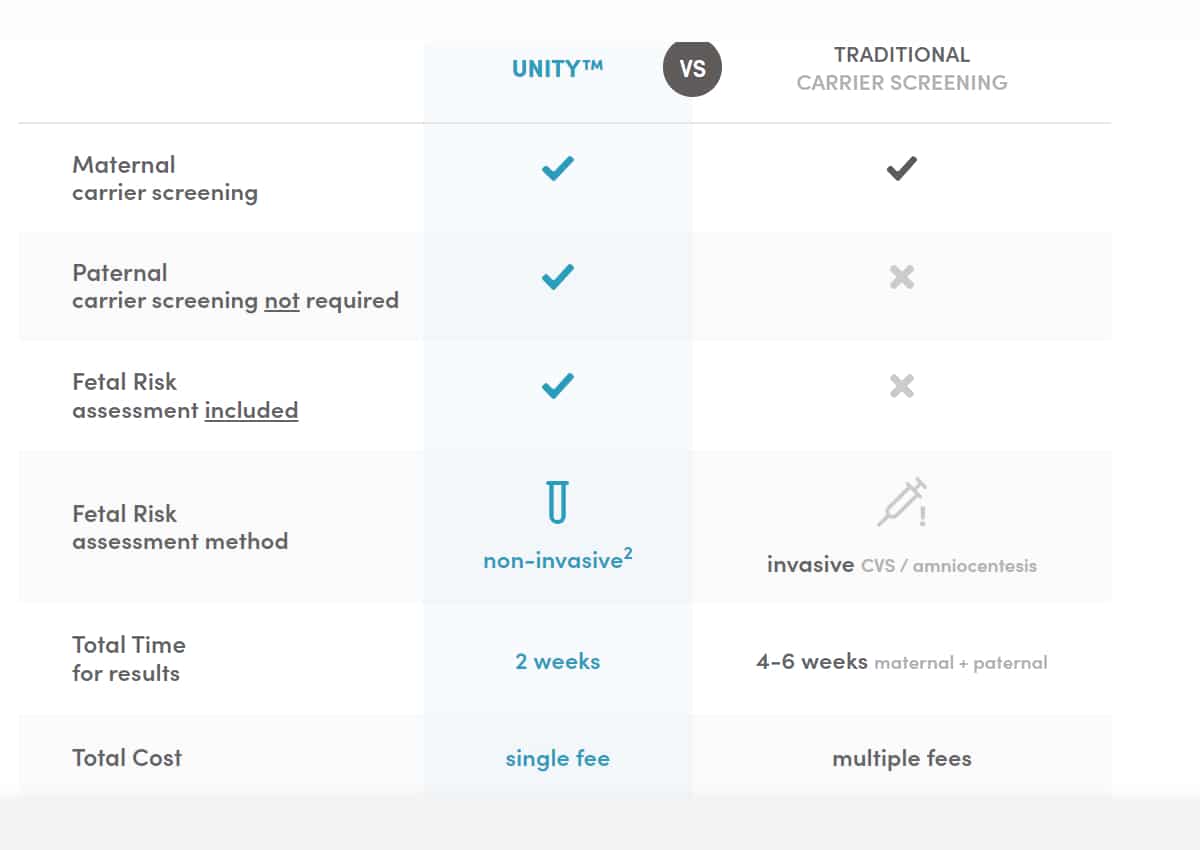
What is the difference between the UNITY screen and traditional carrier screening?
What technology enables the UNITY screen?
Have questions? We have answers.
If your questions are not shown here, please contact us directly.
Will insurance cover geneType UNITY Test?
The test is generally not covered by private health insurance.
How early can the testing be performed during pregnancy?
Patients must be at least 10 weeks gestation or greater for aneuploidy and single gene NIPT.
What is the turnaround time?
UNITY™ aneuploidy NIPT results are returned within seven days of lab receipt. Most UNITY™ carrier screen and single-gene NIPT results are returned within 14 days from lab receipt. If fragile X is ordered, you will receive an additional report(s) shortly after the initial carrier screen result.
What is the UNITY Screen process?
A pregnant patient’s blood can be drawn at 10 or more weeks gestation. From that one sample, UNITY Screen first identifies if the patient is a carrier for specific recessive conditions and if the pregnancy is at risk for common aneuploidies. Foetal aneuploidy results are reported first, within about seven days from lab receipt. If the patient is a carrier, non-invasive prenatal testing will automatically be performed from the original blood sample to determine if the foetus is at risk for those specific recessive conditions. The patient’s carrier status along with the foetal risk is reported together within about two weeks from lab receipt. If RhD antigen screening or additional foetal antigens are ordered, they are reported with the aneuploidy results.
Can UNITY™ be performed in twin pregnancies?
UNITY™ is validated for carrier screening and NIPT for aneuploidy in twin pregnancies. Foetal sexes will be reported if desired and usually we can distinguish foetal sexes between twins. Foetal RhD is also available in twins. If a patient is found to be a carrier on our testing, single gene NIPT is NOT validated in a twin pregnancy.
What makes UNITY Screen different from other NIPTs?
Several non-invasive tests can assess foetal risk for chromosome abnormalities such as aneuploidies. UNITY Screen not only offers screening for these conditions, but it also includes screening for recessive, single-gene disorders. UNITY enables assessing foetal risk for more types of common and severe genetic conditions from one blood draw, from the pregnant patient. UNITY also offers RhD antigen screening to help clarify the need for Rhₒ(D) immune globulin, and, for alloimmunized patients, other red blood cell antigens associated with severe Hemolytic Disease of the Foetus and Newborn (HDFN). UNITY Screen screens for this combination of conditions without requiring a paternal sample at any point in the process.
Which patients is UNITY Screen for?
UNITY Screen may be appropriate for any pregnant patient. The recessive conditions and aneuploidies available on UNITY are recommended for screening in all pregnancies. The optional screening for various red blood cell foetal antigens may be appropriate for specific subsets of pregnant patients.
What genetic conditions does UNITY Screen include?
UNITY Screen includes a combination of recessive, or inherited, conditions and aneuploidies, or chromosome conditions. The key recessive conditions are cystic fibrosis, spinal muscular atrophy, sickle cell disease, alpha thalassemia, and beta thalassemia. Key aneuploidies include trisomy 21, 18, and 13; and four sex chromosome aneuploidies. These conditions are relatively common in the general population.
What is UNITY Screen's foetal antigen NIPT for alloimmunized patients?
About 1% of pregnancies are complicated by alloimmunisation which can lead to Hemolytic Disease of the Foetus and Newborn (HDFN). UNITY Screen’s foetal antigen NIPT looks for the presence of foetal antigens associated with severe HDFN at 10+ weeks into pregnancy. Early, non-invasive screening may significantly streamline management and monitoring for pregnant patients whose foetuses will not express the antigen of concern, allowing healthcare providers to focus resources on pregnancies truly at risk, which is about 15% of the Australian pregnant population.
What is UNITY Screen's foetal RhD NIPT for non-alloimmunized patients?
If a pregnant patient is RhD negative but their partner is RhD positive, the foetus may inherit the RhD positive blood from the partner. About 60% of the babies born to an RhD negative mother and RhD positive father will be RhD positive. Most pregnant patients with an RhD negative blood type are offered a RhoD Immune Globulin treatment, in order to prevent RhD sensitisation as it can lead to Hemolytic Disease of the Foetus and Newborn (HDFN) in future pregnancies. UNITY Screen’s RhD NIPT looks for the presence of foetal RhD antigen at 10+ weeks into pregnancy to help determine which patients are truly at risk for RhD sensitisation. It is expected that 40% of RhD negative pregnant patients may not benefit from RhoD Immune Globulin treatment, therefore, streamlining care for these patients.
What is the difference between UNITY Screen and traditional carrier screening?
Traditional carrier screening looks to see if an individual is a carrier for specific recessive conditions. While carrier screening is recommended to be done preconception, it is most commonly initiated early in pregnancy and done sequentially, with the pregnant patient screened first and then their partner if the pregnant patient is found to be a carrier. If they are both carriers of the same condition, the couple has a 1 in 4 risk to have an affected child with the condition. When a couple is identified as “high-risk” during pregnancy, diagnostic testing is offered. UNITY Screen includes a carrier screen to first understand if the pregnant patient is a carrier, so that part is the same. However, if the pregnant patient is a carrier, UNITY then assesses foetal risk for those conditions automatically, using the original blood sample. The individualised foetal risk score can be as high as 9 in 10 and does not require a paternal sample. This refined risk information can help inform whether or not a diagnostic procedure is recommended.
What technology enables assessing foetal risk for recessive conditions?
Traditional NIPT focuses on assessing foetal risk for larger chromosome changes. However, several common and severe conditions are the result of much smaller genetic changes, within a single gene, that are not as abundant in the maternal bloodstream. BillionToOne’s proprietary molecular counting technology, or Quantitative Counting Templates (QCTs), quantifies DNA molecules to detect conditions caused by tiny DNA variations that can be sparse in a blood sample. These include pregnancy-related DNA that code for recessively inherited conditions. To screen for recessively inherited conditions, a blood sample is collected and these tiny fragments are first amplified. However, amplification can confound the challenge of detecting disease because molecules amplify at different rates. Adding in BillionToOne’s QCTs into the sample prior to amplification provides a measurement tool that allows for better quantification after the amplification step to determine how many disease molecules were in the initial blood sample to begin with which provides an accurate foetal risk assessment.
What technology enables assessing foetal risk for recessive conditions?
A 2022 clinical study of pregnancy outcomes demonstrated UNITY’s single-gene NIPT accurately identified foetuses with recessive conditions as high-risk. This study evaluated the clinical performance of carrier screening coupled with single-gene NIPT for carriers of cystic fibrosis, spinal muscular atrophy, sickle cell disease, alpha-thalassmeia, and beta-thalassemia. The study included 9151 pregnant individuals from 31 US states. 1669 individuals, or 18.2%, were carriers for at least one condition and had UNITY’s single-gene NIPT. Results were compared to prenatal diagnostic testing or newborn outcomes for 191 pregnancies. UNITY’s single-gene NIPT correctly identified 93.3% of affected foetuses as high-risk. The positive predictive value was 48.3%, or nearly two times the maximum positive predictive value of carrier screening when both partners are identified as carriers; which is 1 in 4 or 25%. The negative predictive value was 99.4% meaning single-gene NIPT correctly identified almost all unaffected foetuses as low risk.
Are the conditions on UNITY Screen treatable?
Many of the conditions available on UNITY Screen are treatable. Spinal muscular atrophy, or SMA, is a great example of how early knowledge supports early treatments. One in 10,000 live births in Australia are affected by SMA and the disease is the number one genetic cause of death of babies under two in Australia. There is a narrow window for treatment and early diagnosis is key to maximising the benefit of intervention. At 6 months of age, infants with SMA Type I have already lost 90% of their motor neurons. Two types of approved treatment currently exist in Australia: antisense oligonucleotides and gene therapy. The PBS has heavily subsidised the cost of these treatments. They have the potential to significantly improve function and quality of life. One of the treatments for SMA needs to be started shortly after birth. UNITY Screen’s single-gene NIPT is a way to identify at-risk pregnancies to help inform the need for diagnostic testing during pregnancy without waiting for paternal sample collection. If diagnostic testing confirms the foetus is affected, parents are able to begin preparations before birth. There are also new treatments emerging each year for cystic fibrosis and sickle cell disease, among others. For conditions that are less treatable today, early knowledge assists planning where to deliver the baby, finding support and advocacy, and researching services and forthcoming therapies.
How can I order UNITY™?
Please email us at support@genetype.com. We will send kits to your clinic and provide support.
What’s happening?
GeneType Multi-Risk assessments tailored to your needs
It’s not always easy to know what questions to ask your Doctor. Here are some breast cancer risk questions…
Breast Cancer risk – Starting the conversation
It’s not always easy to know what questions to ask your Doctor. Here are some breast cancer risk questions…
Proactive steps for breast cancer risk reduction
Breast awareness is important whether you are 35 or 75. When you understand your risk, you can be a better advocate for yourself…
References
- The Royal Australian College of General Practitioners. Putting prevention into practice: Guidelines for the implementation of prevention in the general practice setting. 3rd edn. East Melbourne, Vic: RACGP, 2018.
Keep up-to-date with our latest advances
Sign up to our newsletter to stay informed about our latest advances and how these could support your practice.