Get screened based on your personal prostate cancer risk
Current risk assessment for prostate cancer focus on family history.1
However, most men diagnosed with prostate cancer have no significant family history.²
Use geneType for Prostate Cancer to access screening based on your wholistic risk of developing prostate cancer.

Did you know that four key risk factors contribute to your prostate cancer risk?²
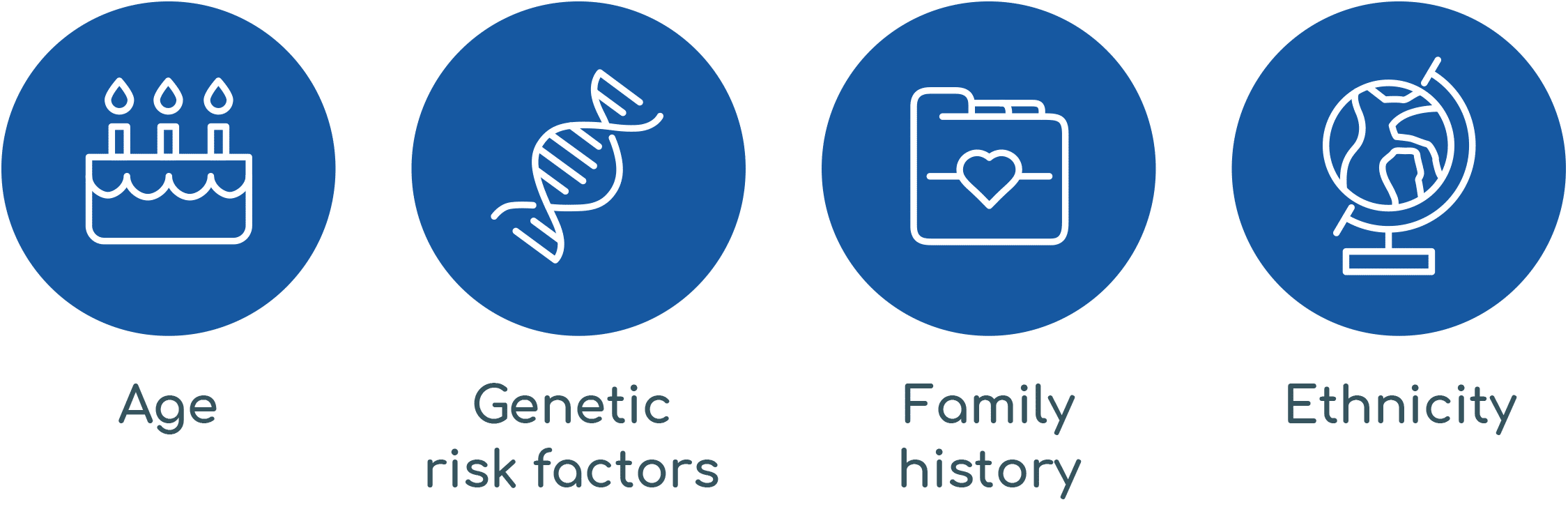

How often should you do a PSA screen?
PSA screening is an effective tool, but the choice to screen is left up to you and your health provider.¹
Have a more informed discussion with your doctor by knowing your wholistic risk of prostate cancer.
The geneType patient
GeneType for Prostate Cancer is appropriate for:
- Men
- Age 30 – 85 years
- No known hereditary gene mutation e.g. BRCA2


Uniquely powered to assess your risk
Get a clearer picture of your risk.
Make screening decisions that fit your needs.
Have questions? We have answers.
If your question is not shown here, please contact us directly.
Will insurance cover this test?
Know your risk of prostate cancer
so you can take action
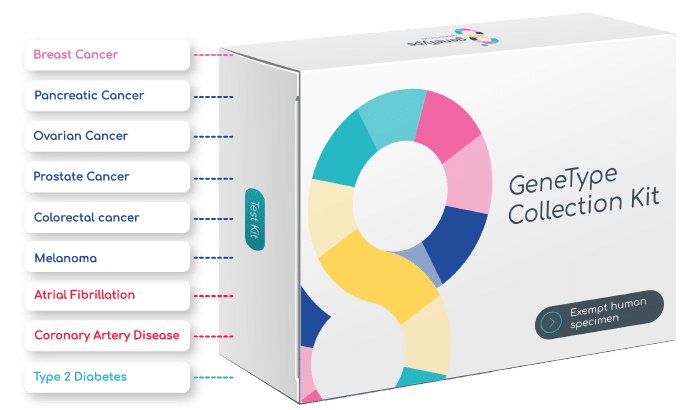
*Patient eligibility dependent on personal medical history, age and sex
Interested in ordering more than one disease? Order geneType Multi-Test.
See individual disease pages for more information about each test.
The Multi-Risk suite of tests is for adults 40-85 years of age. At maximum, a woman would be eligible for 8 diseases in the panel; a man would be eligible for 7. Starting at age 30, a patient may qualify for geneType's cancer risk assessments only.
What’s happening?
Start the conversation about Breast Cancer risk
Under the fluorescent lights of a doctor’s office, it’s not always easy to know what questions to ask. Here are some breast cancer risk questions…
Proactive steps for breast cancer risk reduction
Breast awareness is important whether you are 35 or 75. When you understand your risk, you can be a better advocate for yourself…
GeneType: A new era for Genetic Technologies
Genetic Technologies changed a lot in the last year. Our product innovations, expansion into new therapy areas and acquisitions…
References
1. American Cancer Society Prostate Cancer Screening Recommendations. Accessed June 2022.
2. Rawla P. World J Oncol 2019;10:63–89.







