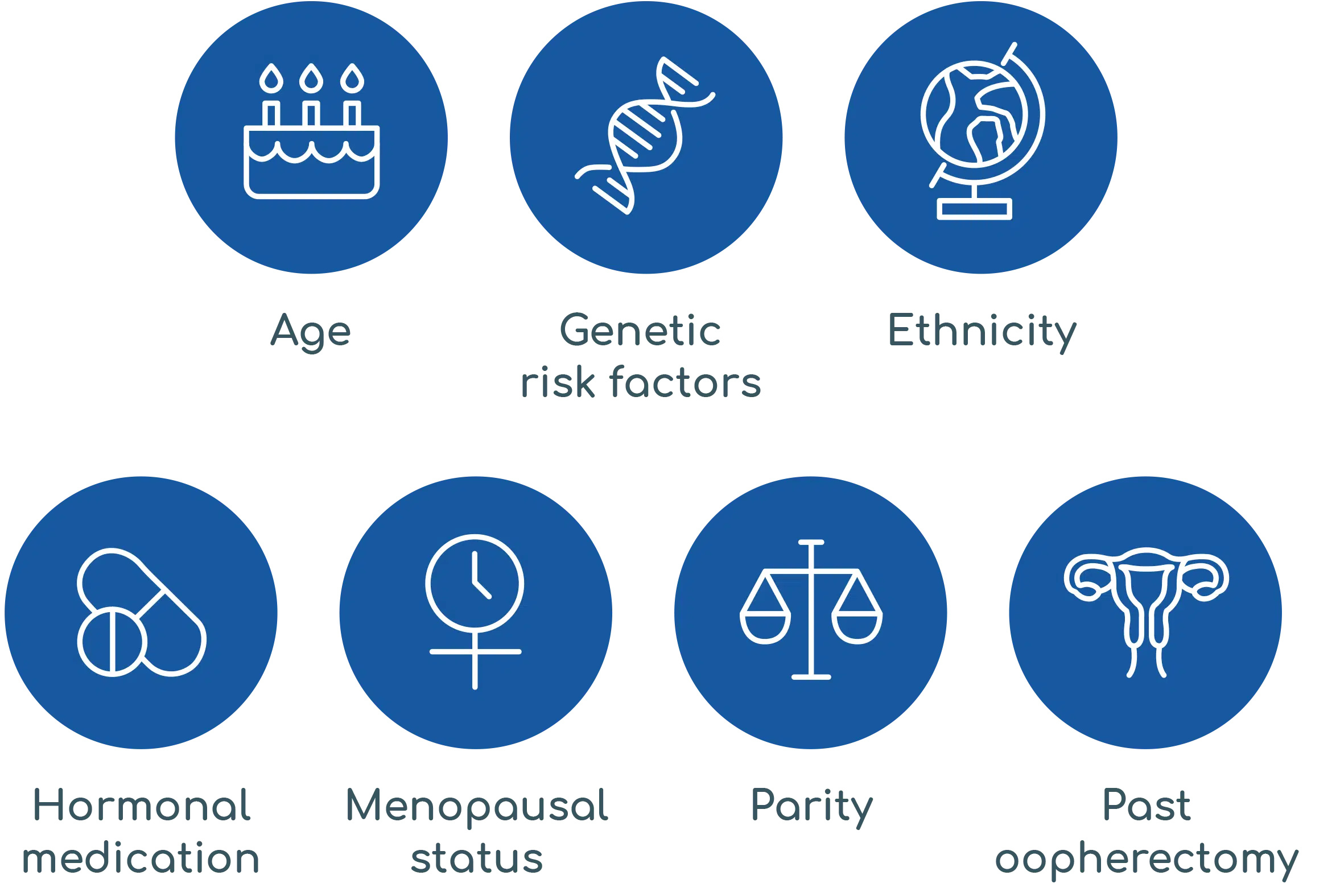
Her risk for ovarian cancer is multi-faceted
How do you review her risk of ovarian cancer?
geneType™ for Ovarian Cancer assesses her holistic risk of ovarian cancer based on her personalised clinical and genetic risk factors including:

geneType™ for Ovarian Cancer assesses her holistic risk of ovarian cancer based on her personalised clinical and genetic risk factors including:

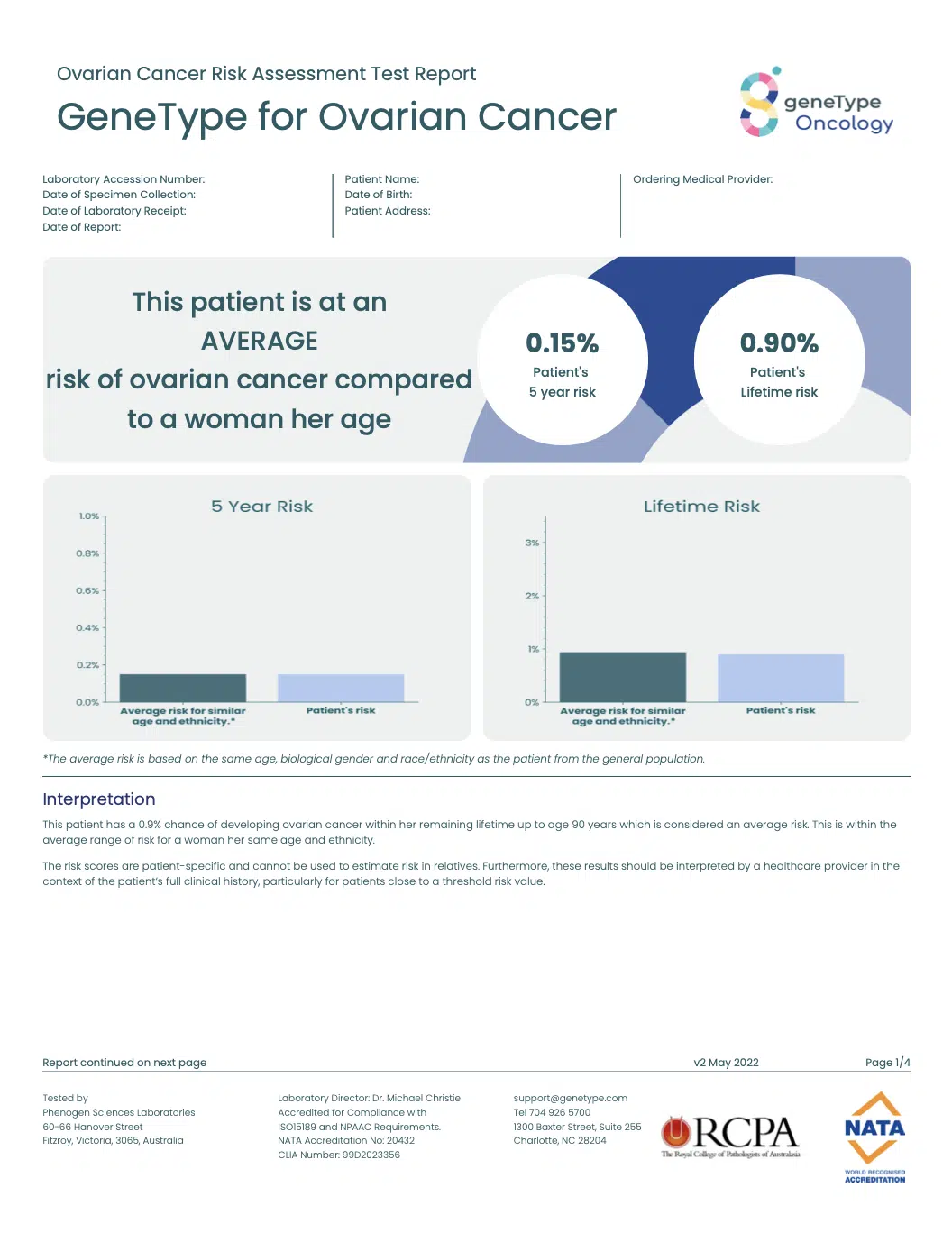
geneType™ reports are structured to make the results easy to interpret and communicate to a patient.
The geneType™ for Ovarian Cancer report includes:

geneType™ for Ovarian Cancer is appropriate for:

Register as a provider; our team will provide clinical education and other resources as needed.
We will send kits to your clinic to have on hand.

Discuss geneType™ with your patient.
Would geneType™ help them qualify for additional risk reduction strategies?

Complete the Test Requisition form, collect a saliva sample from the patient with the collection kit provided and return both to Rhythm.
You will have the option to use a paper requisition, or a secure, compliant portal to complete the ordering process.

Leave the rest to us.
We will notify you when your patient’s results are ready.
You will have the option to request a consult with a genetic counsellor, whether to review your patient’s results and/or to follow-up with your patient, as needed.
If your question is not shown here, please Contact us directly.
No. At this time geneType™ is a self-pay test. However, your patient may use FSA/HSA to pay for the test.
No. This risk assessment does not test for hereditary breast and ovarian cancer (HBOC), or any other hereditary cancer syndrome. geneType™ is a risk assessment for asymptomatic women in the general population who are either ineligible for HBOC testing, or who have come back as negative carriers following HBOC testing.
geneType™ for Ovarian Cancer is suitable for women aged 40 years or older.
No. This test is not applicable to women who have a personal history of ovarian cancer or who have already been shown to have an HBOC mutation, for example in the BRCA1 or BRCA2 gene, or a diagnosis of a genetic syndrome that may be associated with elevated risk of ovarian cancer.
geneType™ is a risk assessment tool to enable you to stratify your patient population. Liquid biopsies, such as the Galleri test are novel, but pricey screening options. A risk stratification tool can help identify your patients that might benefit from a novel screening tool like this one given the paucity of effective early detection tools available.
*Patient eligibility dependent on personal medical history, age and sex
The Multi-Risk suite of tests is for adults 40-85 years of age. At maximum, a woman would be eligible for eight diseases in the panel; a man would be eligible for seven. Starting at age 30, a patient may qualify for geneType’s™ cancer risk assessments only.
1. US Preventive Services Task Force. JAMA 2018;319(6):588–594.
2. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2022 — March 9, 2022.

Accredited For Compliance With NPAAC Standards And ISO 15189