Most breast cancers are diagnosed in women with no significant family history
There are a variety of risk factors that may influence your overall risk of breast cancer including:
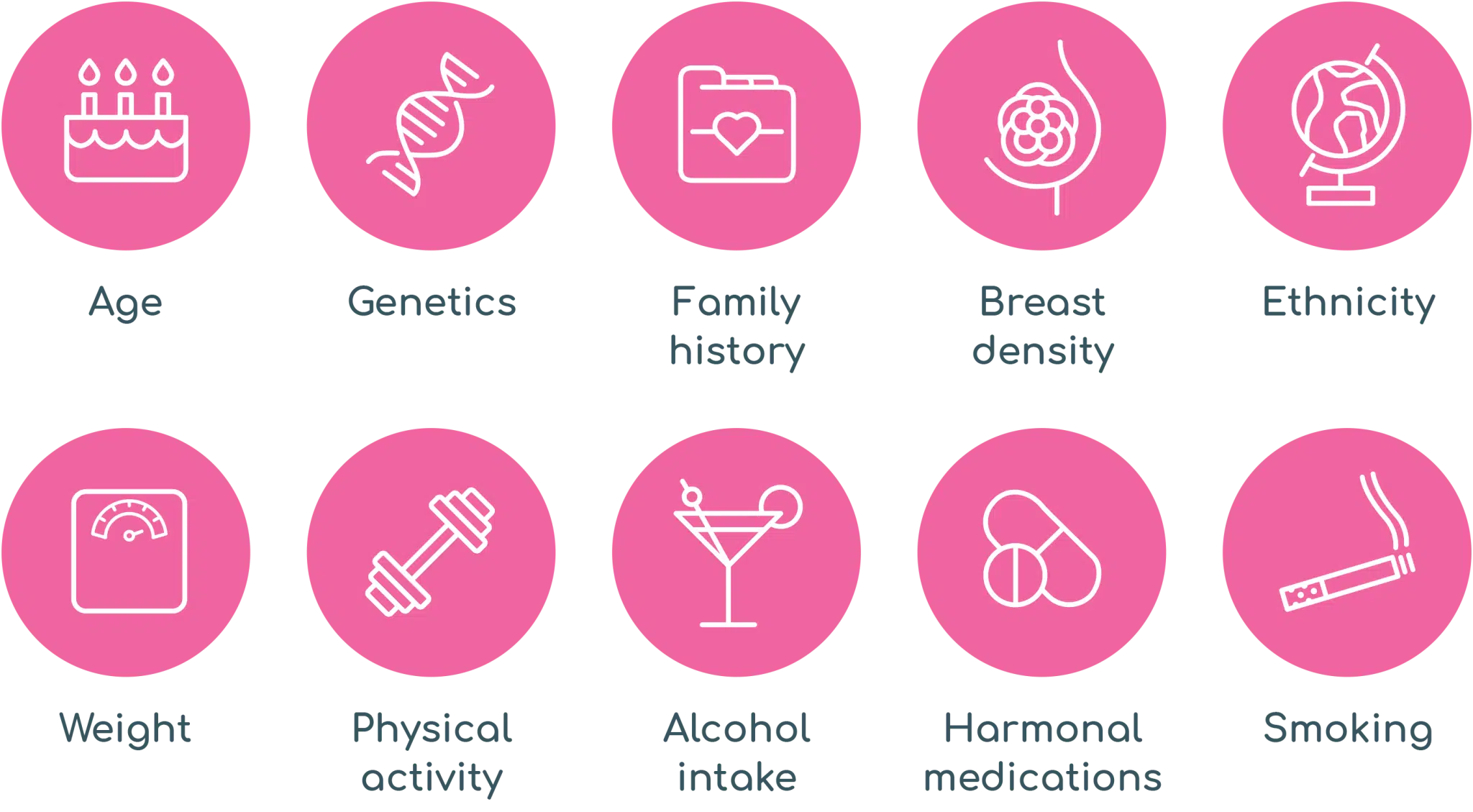
There are a variety of risk factors that may influence your overall risk of breast cancer including:

Learn more about our breast cancer test with this video featuring Dr. Carolynn Young and Dr. Joel Evans discussing factors that influence breast cancer risk and the clinical utility of geneType™ for Breast Cancer on The Balancing Act.
geneType™ for Breast Cancer can help you and your healthcare provider develop a risk reduction plan just for you.
Depending on your risk, your healthcare provider may discuss some or all of the following:
Is geneType™ for Breast Cancer
right for you?
On their own, each risk factor adds only a small amount of risk to your chances of developing breast cancer.
Together, they may increase your risk to a point where a proactive approach is recommended.
| I am 30 or older | |
| I have been told I have dense breast tissue | |
| I have not had a mammogram within the past year | |
| I have a relative who has had breast cancer | |
| I have taken (am taking) oral contraception | |
| I gave birth to my first child after 30 | |
| I did not breastfeed | |
| I have been taking hormone replacement therapy | |
| I have started menopause | |
| I’ve gained post-menopausal weight | |
| I want to understand my “baseline” genetic risk |

geneType™ for Breast Cancer is the only test that gives you a comprehensive and highly accurate risk prediction score based on:
By looking at common markers of risk in your genes, we get a better picture of disease risk. We call this risk factor, gene risk.
Everyone has these markers, but not everyone has the same combination. Like a fingerprint, your genetic markers are unique to you, and can help define your “baseline” risk of developing breast cancer.

Register for your geneType™ test using our portal.
Depending on the test, you may require access to your medical records (ex. lipid panel or breast density results).
Payment will be collected at time of registration.

Your clinical information will be reviewed by our third party telehealth partner, DNA Visit.
If you qualify for testing, a kit will be shipped to your address on file.
If you do not qualify for testing, you will receive a refund

Complete buccal sample collection as instructed and return in pre-paid envelope.
Do not eat, drink, smoke, vape or chew gum within 30 minutes of collection.

Step 4
When your results are ready, you will have the chance to speak with your DNA Visit healthcare provider.
All at-risk patients will be required to speak with the provider prior to results-release.
Turn-around time is 10-12 weeks.
If your questions are not shown here, please Contact us directly.
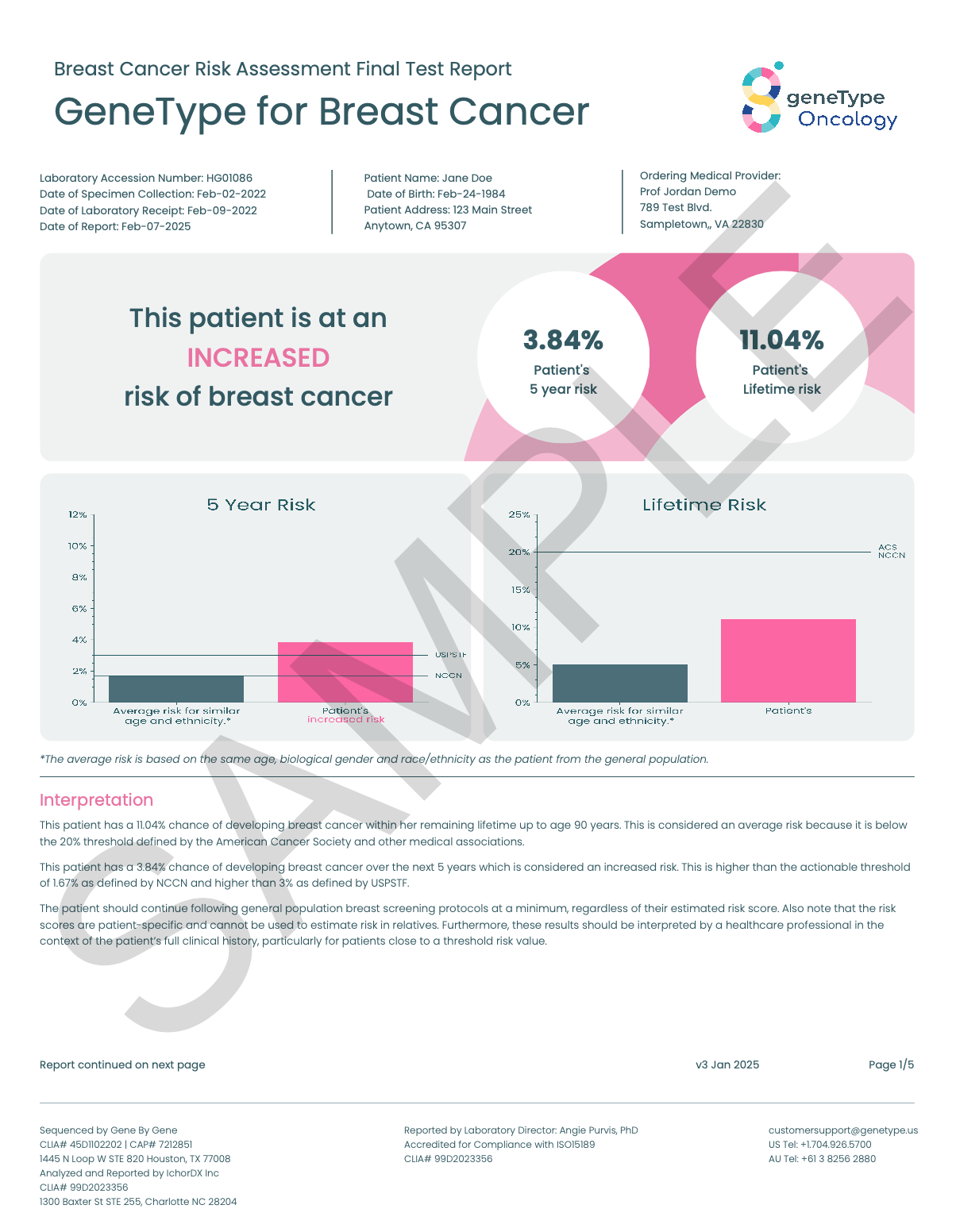
Your results will tell you, based on genetics and other risk factors, your risk for developing sporadic breast cancer over two different time periods: in the next 5 years and in your remaining lifetime.
You and your healthcare provider can use this information to develop a personalized screening and risk reduction plan
No! This risk assessment is for women either do not qualify for hereditary testing OR who have already tested negativefor hereditary pathogenic variants (aka mutations) in genes like BRCA1/2. The majority of women will not be carriers of these types of hereditary mutations, but will still be at risk of developing breast cancer. geneType™ helps to better identify the women that are at risk of “flying under the radar.” To understand your risk of developing sporadic (non-hereditary) breast cancer, geneType™ can help.
Great work maintaining your routine mammogram screening!
Your breast cancer risk score can also help you and your healthcare provider develop a personalized screening plan for you, which may involve additional screening than just mammogram alone.
No!
Your geneType™ for Breast Cancer results will indicate whether you are at increased risk, or average risk. If you are at average risk, it is still important to follow the recommended screening guidelines for women of your age.
geneType™ for Breast Cancer is available for women of all backgrounds. The risk model incorporates ethnicity-specific polygenic risk scores and ethnic-specific population incidence data derived from the Surveillance, Epidemiology, and End Results Program (SEER).
If you ordered geneType™ through your own health provider, you will receive your results directly from them.
Yes!
You can order through our third party telehealth provider in a few simple steps.
1. Register on our patient portal by clicking any Order now button on our site.
3. Enter your information, select the diseases you want, and fill out the clinical questions.
2. Pay for the test.
4. Once the telehealth provider approves your information, you will be sent a geneType™ test.
5. Follow the instructions to collect your DNA with a saliva tube.
6. Return your saliva sample in the mail and wait for results.
7. Review next steps with your telehealth provider.
This test is not generally covered by insurance. However, you may use FSA/HSA to pay for the test. Your ordering healthcare provider will discuss the cost of the test with you. A credit card authorization form will be included in the test kit that lists the payment options. Or you will be able to pay via the geneType™ patient portal.
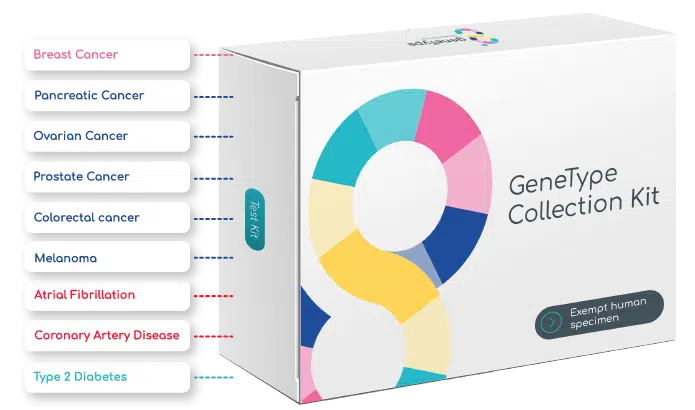
*Patient eligibility dependent on personal medical history, age and sex
The Multi-Risk suite of tests is for adults 40-85 years of age. At maximum, a woman would be eligible for eight diseases in the panel; a man would be eligible for seven. Starting at age 30, a patient may qualify for geneType’s™ cancer risk assessments only.
1. American Cancer Society. URL: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html
2. van der Groep P et al. Cell Oncol (Dordr) 2011;34:71 -88.
3. Dite GS et al. Cancer Epidemiol Biomarkers Prev 2016;25:359 -65.
4. Allman R, et al. SNPs and breast cancer risk prediction for African American and Hispanic women. Breast Cancer Res Treat. 2015 Dec;154(3):583-9.
5. Spaeth E, et al. Validation of abridged breast cancer risk assessment model for the general population [abstract]. In: Proceedings of the 2021 San Antonio Breast Cancer Symposium; 2021 Dec 7-10; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2022;82(4 Suppl):Abstract nr P2-10-06..
6. Medication Use to Reduce Risk of Breast Cancer, United States Preventative Services Task Force. JAMA. 2019; 322(9):857-867
7. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines); Breast Cancer Screening and Diagnosis. Version 1.2021- April 2022.
8. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines); Breast Cancer Risk Reduction. Version 1.2022 – April 2022
9. Use of Endocrine Therapy for Breast Cancer Risk Reduction. Journal of Clinical Oncology 37, no. 33 (November 20, 2019) 3152-3165.
10. Monticciolo D et al. Breast Cancer Screening Recommendations Inclusive of All Women at Average Risk: Update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021 Sep;18(9):1280-1288.

Accredited For Compliance With NPAAC Standards And ISO 15189